This is from a series of articles reprinted from the journal ISA Transactions. All ISA Transactions articles are free to ISA members, or can be purchased from Elsevier Press.
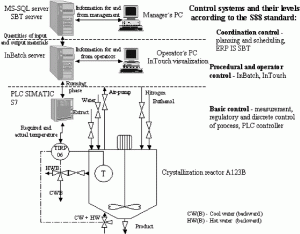
Abstract: This paper describes a case study of a control system design for a batch pharmaceutical process. The ISA standard ISA-88 batch control models and terminology were used as the main guidelines for the implementation. As the ISA-88 is not a guide for how to apply the definitions/structures, etc., one of the main goals of our work was to create a methodology for decomposition of functional requirements in terms of ISA-88 models and structures. This methodology was tested on a real problem, described in the case study. Also presented are some remarks on project methodology and Food and Drug Administration validation.
Free Bonus: To read the full article on batch control for a pharmaceutical plant, click here.
ISA membership entitles you to free access to all ISA Transactions articles plus a wealth of technical content, industry information, free webinars, training opportunities, program discounts, certification and licensure and professional networking.
Join ISA ... learn, advance, succeed!