The following insights are part of an occasional series authored by Greg McMillan, industry consultant, author of numerous process control books and a retired Senior Fellow from Solutia Inc., now Eastman Chemical.
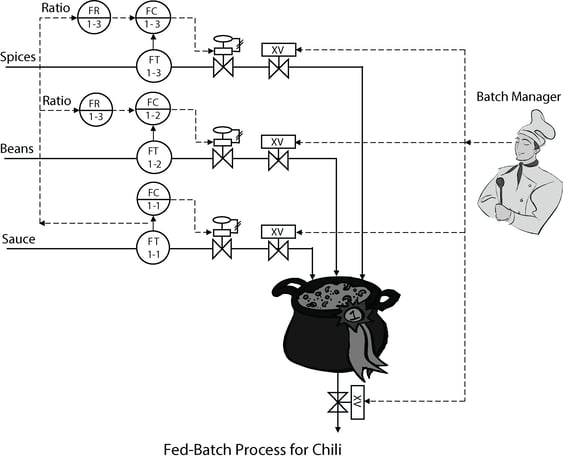
In pure batch operations, changes in feed conditions, disturbances, or demand show up as changes in the profile of key process variables. For a bioreactor, variability in the seed culture, indeterminacy of cell response and changes in the demand for air, substrate (e.g. glucose), and reagent cause the variation in the batch profile for dissolved oxygen and substrate concentration and pH. Process engineers can be ingenious at changing the timing and flow rate of air, substrate, and reagent via sequential scheduling. However process knowledge is far from perfect and unknowns change the profiles of the key process variables. Feedback control is needed because the process changes from minute to minute and the process response is not totally repeatable besides not being entirely predictable. Even model predictive control relies on feedback control to bias the predicted future response trajectory.
Insight: The sequential scheduling of batch feeds in pure batch operations presumes complete knowledge and a lack of variability in raw materials and batch behavior.
The fed-batch control system enables the dissolved oxygen and substrate concentration and pH to be more constant throughout the batch. The variability in these controlled variables is transferred to the manipulated air, substrate, and reagent flows. At first this can be disquieting because operations personnel have now lost the ability to force these flows to follow a fixed pattern.
The definition of a controlled variable and a manipulated variable is based on the latter serving the needs of the former and not vice versa. The final resting value of manipulated variables can be optimized by slowly adjusting controlled variables. This is in fact the strategy of valve position controllers that seek to increase production rate by keeping a coolant valve at the maximum controllable position by manipulating a feed flow. One cannot keep constant both the controlled variable and the manipulated variable.
Insight: The feedback control key process variables by the manipulation of batch feeds in fed-batch will automatically correct for unknowns and disturbances.
The optimized fed-batch control system makes adds a higher mode of control. A model predictive controller whose controlled variables are the slope of the biomass concentration profile (cell growth rate) and the slope of the product concentration profile (product formation rate) manipulates the dissolved oxygen and substrate concentration setpoints. The result is a batch profile of these higher controlled variables that more closely matches their optimums.
Insight: The addition of an upper mode of control by the addition of changes of compositions and temperature with respect to time as controlled variables can optimize batch profiles.
For a given size reactor, the production rate is higher for a continuous reactor than for a pure batch or fed-batch reactor. After startup a continuous reactor is continually producing whereas a pure batch or fed-batch reactor takes time to charge reactants, bring the contents to the proper temperature and pressure, wait for the reaction to complete, and then empty the reactor. In some cases the reactor may need to wait on shared resources such as feed weigh tanks or on operator actions or lab results from sequential actions.
Batch reactors are particularly vulnerable to an attitude that every flow must be scheduled based on process knowledge. The use of feedback control to automatically set these flows based on actual process response is critical. The addition of successively higher level of control transfers variability from more important process outputs to the flows that are process inputs. See Greg McMillan's ISA book, Advances in Reactor Measurement and Control, for an extensive view of practical opportunities for designing control strategies to achieve product quality and maximize yield and capacity in different types of reactors.
Continuous processes cost less in terms of investment and utilities for a given capacity but generally require greater process research, development, and design. Mature high capacity products (e.g., oil, gas, and petrochemicals) tend to use continuous reactors whereas new high value processes (e.g., specialty chemical and biological) primarily use batch and fed-batch reactors. As volumes increase and the profit margins decrease (the product becomes a commodity), there is increased emphasis on developing a continuous process to provide a higher capacity and lower operating cost.
Candidates for continuous reactors are products with a low profit margin, a high volume requirement, fast reactions, minimal adverse reactions, preventable buildup of inhibitors and inactive components, and extensive Research and Development (R&D) history leading to a deep fundamental knowledge of kinetics. Oil, gas, chemical intermediates, petrochemicals, and commodity chemicals use continuous reactors. Extensive integration of unit operations for energy recovery and recycle of materials offers complex opportunities for optimization.
Insight: Continuous reactors offer more capacity for a given size or a lower capital cost and greater energy efficiency for a given capacity but require greater knowledge and effort in research, development and design.
The fastest and simplest implementation is pure batch with quantities charged sequentially mimicking lab experiments much like ingredients in a recipe. As knowledge is gained batch reactors can move to become fed-batch reactors and eventually continuous reactors if there is enough demand and if reaction chemistry permits a variable residence time.
In contrast to continuous reactors candidates for batch reactors are products with a high profit margin, low volume requirement, slow reaction, significant side effects, and minimal knowledge of kinetics. Specialty chemicals, food, beverages, and especially biopharmaceuticals are produced by batch reactors. For new biopharmaceuticals with the patent expiration clock ticking and extraordinarily high prices (e.g. > $1000 per gram), the time to market supersedes any consideration of process efficiency or capital cost. These processes have extremely slow kinetics, requiring days to weeks for the cells to produce product.
Insight: Batch operations require less process knowledge and customization of equipment and offer a fixed and tight residence time distribution enabling a more certain result and a faster project when a new product is commercialized.
The buildup of dead and mutated cells, toxins, inhibitors, viruses, and bacteria can be prevented in batch processes by emptying, cleaning, and sterilizing the equipment and piping after each batch. The adverse results of contamination to food, beverages, and drugs can be extreme in terms of the loss of capacity, cost of retrieval, and most of all the consequences to the customers.
Insight: Batch processes enable the periodic emptying, cleaning and sterilization of equipment and piping to prevent the buildup of contaminants in products that end up in a person or animal.
Some mature biopharmaceuticals are produced by “perfusion” processes operating in the continuous mode for months at a time with extensive recycle due to the slow kinetics. Product is removed from the discharge stream and the recycle stream is purified before being returned to the bioreactor. These processes are periodically shut down for total elimination of mutated cells, inhibitors and alien organisms and decontamination.
R&D and process design will determine whether a reactor will be batch or continuous based on process knowledge, product capacity requirements, profit margin, and impact of time to market. Once this decision is made, the process control engineer should promote the automatic transfer of variability from the successively higher loops to lower loops. Essential is the understanding that variability must be transferred or absorbed and cannot simply entirely disappear and processes inherently have a significant degree of non-repeatability and unpredictability.

About the Author Gregory K. McMillan, CAP, is a retired Senior Fellow from Solutia/Monsanto where he worked in engineering technology on process control improvement. Greg was also an affiliate professor for Washington University in Saint Louis. Greg is an ISA Fellow and received the ISA Kermit Fischer Environmental Award for pH control in 1991, the Control magazine Engineer of the Year award for the process industry in 1994, was inducted into the Control magazine Process Automation Hall of Fame in 2001, was honored by InTech magazine in 2003 as one of the most influential innovators in automation, and received the ISA Life Achievement Award in 2010. Greg is the author of numerous books on process control, including Advances in Reactor Measurement and Control and Essentials of Modern Measurements and Final Elements in the Process Industry. Greg has been the monthly "Control Talk" columnist for Control magazine since 2002. Presently, Greg is a part time modeling and control consultant in Technology for Process Simulation for Emerson Automation Solutions specializing in the use of the virtual plant for exploring new opportunities. He spends most of his time writing, teaching and leading the ISA Mentor Program he founded in 2011.
Gregory K. McMillan, CAP, is a retired Senior Fellow from Solutia/Monsanto where he worked in engineering technology on process control improvement. Greg was also an affiliate professor for Washington University in Saint Louis. Greg is an ISA Fellow and received the ISA Kermit Fischer Environmental Award for pH control in 1991, the Control magazine Engineer of the Year award for the process industry in 1994, was inducted into the Control magazine Process Automation Hall of Fame in 2001, was honored by InTech magazine in 2003 as one of the most influential innovators in automation, and received the ISA Life Achievement Award in 2010. Greg is the author of numerous books on process control, including Advances in Reactor Measurement and Control and Essentials of Modern Measurements and Final Elements in the Process Industry. Greg has been the monthly "Control Talk" columnist for Control magazine since 2002. Presently, Greg is a part time modeling and control consultant in Technology for Process Simulation for Emerson Automation Solutions specializing in the use of the virtual plant for exploring new opportunities. He spends most of his time writing, teaching and leading the ISA Mentor Program he founded in 2011.