The following technical discussion is part of an occasional series showcasing the ISA Mentor Program, authored by Greg McMillan, industry consultant, author of numerous process control books, 2010 ISA Life Achievement Award recipient and retired Senior Fellow from Solutia Inc. (now Eastman Chemical). Greg will be posting questions and responses from the ISA Mentor Program, with contributions from program participants.
In the ISA Mentor Program, I am providing guidance for extremely talented individuals from countries such as Argentina, Brazil, Malaysia, Mexico, Saudi Arabia, and the USA. This question comes from Danny Parrott.
Danny Parrott is an instrumentation and controls specialist at Spallation Neutron Source. Danny is a detail-oriented instrumentation and controls professional experienced in the areas of electrical, electronics and controls specification, installation, maintenance, and project planning. Danny's question is important in dealing with the many challenges for reliable and accurate pH measurement in ultra pure water and more generally in streams with exceptionally low conductivity.
Danny Parrott’s Question
What are some opinions, thoughts, or practical experience relating to pH measurements in ultra-pure water applications? What are some opinions, thoughts, or practical experience relating to pH measurements in ultra-pure water applications?
Greg McMillan’s Answer
Ultra-pure water applications pose special problems because of the exceptionally low conductivity of the fluid from the absence of ions. The consequences are extreme sensitivity to fluid velocity and spurious ions, unstable reference junction potentials, sample contamination, and loss of electrical continuity between the reference and measurement electrodes. The functional electrical diagram showing resistances and potentials provides insightful view of nearly all of the sources of problems with pH measurements in general.
Ultra-pure water and process fluids with an exceptionally small near zero fluid conductivity threaten the continuity of the electrical circuit between the reference and measurement electrode terminals at the transmitter by an extraordinarily large electrical resistance R8 in Figure 1, a pH electrode functional electrical circuit diagram for a combination electrode that is a great way of recognizing the many potential source of errors in a pH measurement. Ultra-pure water applications pose special problems because of the exceptionally low conductivity of the fluid from the absence of ions.
The consequences are extreme sensitivity to fluid velocity and spurious ions, unstable reference junction potentials, sample contamination, and loss of electrical continuity between the reference and measurement electrodes. The functional electrical diagram showing resistances and potentials provides insightful view of nearly all of the sources of problems with pH measurements in general. Ultra-pure water and process fluids with an exceptionally small near zero fluid conductivity threaten the continuity of the electrical circuit between the reference and measurement electrode terminals at the transmitter by an extraordinarily large electrical resistance R8 in Figure 1, a pH electrode functional electrical circuit diagram for a combination electrode that is a great way of recognizing the many potential source of errors in a pH measurement.
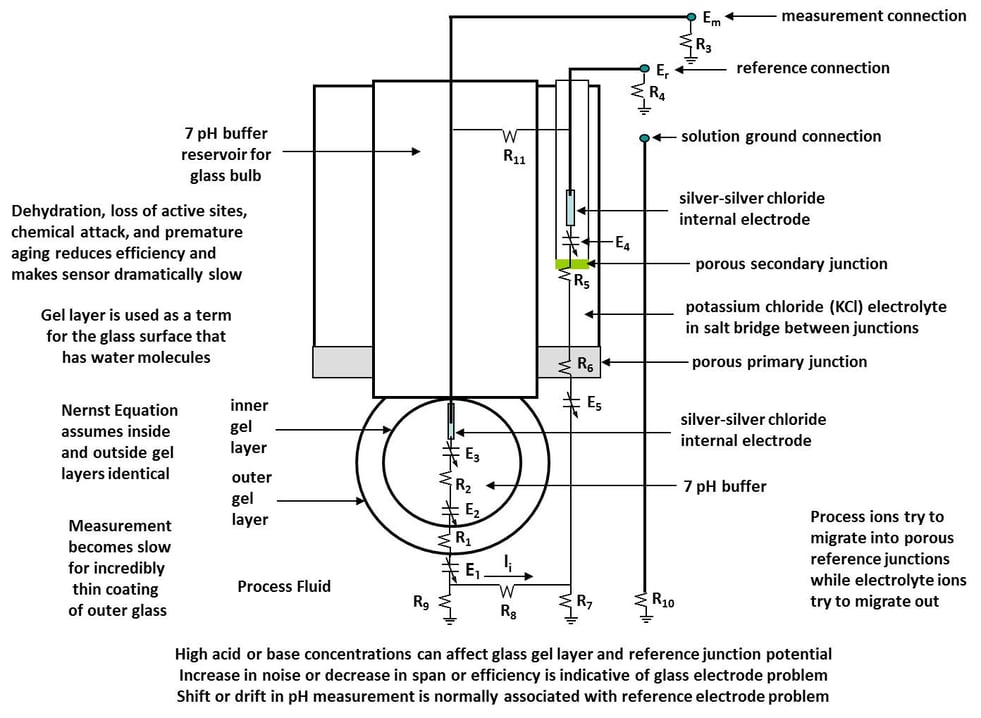 Figure 1: pH Electrode Functional Electrical Circuit Diagram
Figure 1: pH Electrode Functional Electrical Circuit Diagram
The solution for online measurements is to use a flowing junction reference electrode to provide a small fixed liquid junction potential in a low flow assembly for a combination electrode. The combination electrode assembly ensures a short fixed distance path of reference electrolyte to the measurement electrode and a small fixed fluid velocity. The assembly also provides mounting of an electrolyte reservoir that sustains a small fixed reference junction flow as shown in Figure 2. The flow of reference electrode electrolyte reduces the fluid velocity and electrical resistance (R8) in the fluid path and provides a much more constant liquid junction potential (E5) that does not jump or shift due to the appearance of spurious ions. The resistances and potentials in the diagram provide a wealth of information.
The flow assembly also has a special cup holder for calibration with buffer solutions. A solution ground connection reduces the effect of ground potentials. Temperature compensation must be accurate and fast. The solution for online measurements is to use a flowing junction reference electrode to provide a small fixed liquid junction potential in a low flow assembly for a combination electrode. The combination electrode assembly ensures a short fixed distance path of reference electrolyte to the measurement electrode and a small fixed fluid velocity. The assembly also provides mounting of an electrolyte reservoir that sustains a small fixed reference junction flow as shown in Figure 2.
The flow of reference electrode electrolyte reduces the fluid velocity and electrical resistance (R8) in the fluid path and provides a much more constant liquid junction potential (E5) that does not jump or shift due to the appearance of spurious ions. The resistances and potentials in the diagram provide a wealth of information. The flow assembly also has a special cup holder for calibration with buffer solutions. A solution ground connection reduces the effect of ground potentials. Temperature compensation must be accurate and fast.
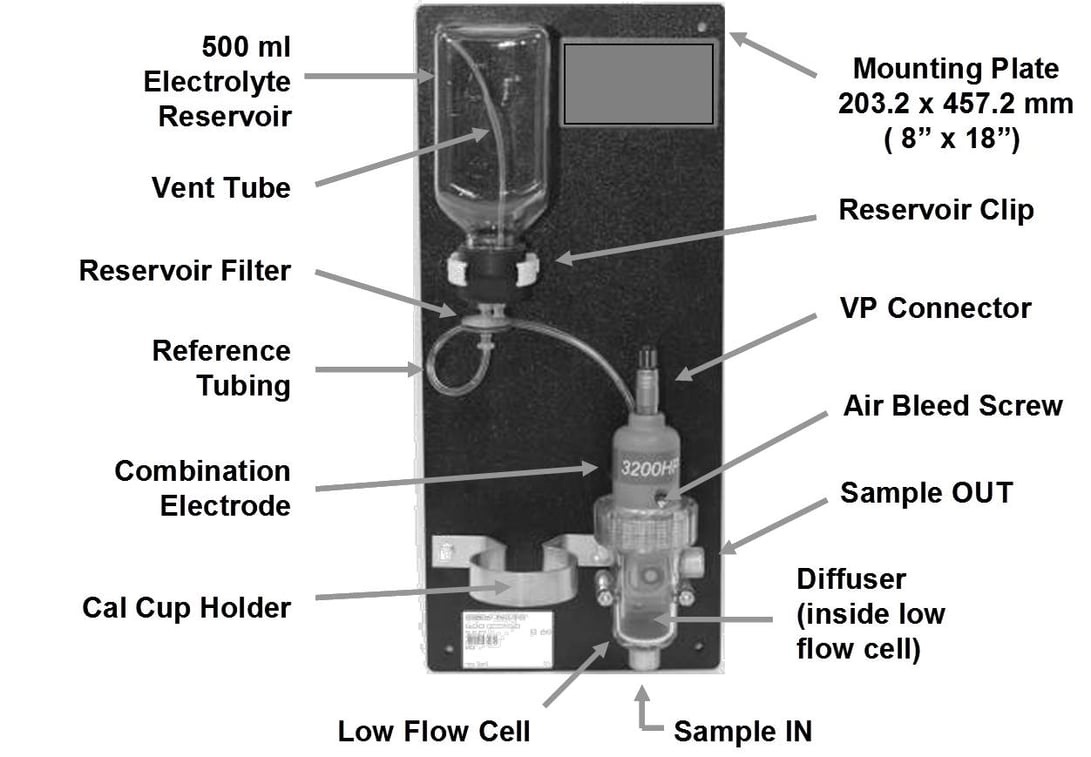 Figure 2: Low Flow Assembly With Flowing Reference Junction for Low Conductivity pH Applications
Figure 2: Low Flow Assembly With Flowing Reference Junction for Low Conductivity pH Applications
The pH measurement calibration needs to be checked and adjusted before installation and periodically thereafter by inserting the electrode(s) in buffer solutions. Making a pH measurement of a sample is very problematic because of contamination from glass beaker ions, absorption of carbon dioxide creating carbonic acid and accumulation of electrolyte ions from flowing junction. The sample volume needs to be large and the measurement made quickly to reduce effect of accumulating ions. A closed plastic sample container is employed to minimize contamination. The same type of electrode(s) in the online measurement should be utilized for sample measurement so that reference junction potentials are consistent. Since these sample pH measurement requirements are rarely satisfied, buffers instead of process samples should be used for calibration checks.
Join the ISA Mentor Program
The ISA Mentor Program enables young professionals to access the wisdom and expertise of seasoned ISA members, and offers veteran ISA professionals the chance to share their wisdom and make a difference in someone’s career. Click this link to learn more about how you can join the ISA Mentor Program.
In exceptionally low conductivity process fluids, there is often not enough water content to keep the glass measurement electrode hydrated. Also, the activity of the hydrogen ion is severely decreased by the lack of water and the extremely different dissociation constant of a non-aqueous solvent can cause a pH range that is outside of the normal 0 to 14 pH range. For these applications, a flowing reference electrode is also needed but an automatically retractable insertion assembly is useful to periodically retract, flush, soak and calibrate the electrodes reducing process exposure time and hydrating/rejuvenating the measurement electrode’s glass surface. For more on the challenges of semi-aqueous pH measurements see the Control Talk article The wild side of pH measurement. For a much more complete view of what is needed for pH applications, see the ISA book Advanced pH Measurement and Control.
For pH measurements used for process control, I recommend three pH assemblies and middle signal selection. Lower lifecycle costs from less and more effective maintenance and better process performance more than pays for cost of the three measurements. Middle signal selection will inherently ignore a single measurement failure of any type and dramatically reduce the effect of spikes, noise, and the consequences of slow or insensitive glass electrodes. The middle selection also eliminates unnecessary calibration checks and provides much more intelligent knowledge on electrode performance enabling optimum time for calibration and replacement.

Additional Mentor Program Resources
See the ISA book 101 Tips for a Successful Automation Career that grew out of this Mentor Program to gain concise and practical advice. See the InTech magazine feature article Enabling new automation engineers for candid comments from some of the original program participants. See the Control Talk column How to effectively get engineering knowledge with the ISA Mentor Program protégée Keneisha Williams on the challenges faced by young engineers today, and the column How to succeed at career and project migration with protégé Bill Thomas on how to make the most out of yourself and your project. Providing discussion and answers besides Greg McMillan and co-founder of the program Hunter Vegas (project engineering manager at Wunderlich-Malec) are resources Mark Darby (principal consultant at CMiD Solutions), Brian Hrankowsky (consultant engineer at a major pharmaceutical company), Michel Ruel (executive director, engineering practice at BBA Inc.), Leah Ruder (director of global project engineering at the Midwest Engineering Center of Emerson Automation Solutions), Nick Sands (ISA Fellow and Manufacturing Technology Fellow at DuPont), Bart Propst (process control leader for the Ascend Performance Materials Chocolate Bayou plant) and Daniel Warren (senior instrumentation/electrical specialist at D.M.W. Instrumentation Consulting Services, Ltd.).
About the Author
Gregory K. McMillan, CAP, is a retired Senior Fellow from Solutia/Monsanto where he worked in engineering technology on process control improvement. Greg was also an affiliate professor for Washington University in Saint Louis. Greg is an ISA Fellow and received the ISA Kermit Fischer Environmental Award for pH control in 1991, the Control magazine Engineer of the Year award for the process industry in 1994, was inducted into the Control magazine Process Automation Hall of Fame in 2001, was honored by InTech magazine in 2003 as one of the most influential innovators in automation, and received the ISA Life Achievement Award in 2010. Greg is the author of numerous books on process control, including Advances in Reactor Measurement and Control and Essentials of Modern Measurements and Final Elements in the Process Industry. Greg has been the monthly "Control Talk" columnist for Control magazine since 2002. Presently, Greg is a part time modeling and control consultant in Technology for Process Simulation for Emerson Automation Solutions specializing in the use of the virtual plant for exploring new opportunities. He spends most of his time writing, teaching and leading the ISA Mentor Program he founded in 2011.
Connect with Greg:




