This post was written by Jonathan Lustri is a life sciences industry consultant for Emerson Automation Solutions.
The pharmaceutical manufacturing industry has had to work within the constraints of regulation for many decades, which has inhibited a culture of continuous process improvement and caused less than optimal manufacturing practices. There is a reluctance to change a validated process that may require regulatory approval. The Food and Drug Administration (FDA) initiative for current good manufacturing practices (CGMPs) for the twenty-first century has encouraged the adaption of new technologies to improve manufacturing. Process analytical technology (PAT) is one of these technologies. This post presents some of the challenges and best practices related to implementing PAT and gives a perspective on lessons learned.

The traditional approach to quality within the pharmaceutical industry is to perform post-quality-control (QC) testing to verify the batch has met critical quality specifications. If quality specifications are not met, the batch is scrapped. Manufacturers are moving away from this approach toward a quality-by-design approach by combining a higher level of process understanding with more sophisticated real-time monitoring and control.
The FDA has sought to encourage the industry to make advancements in manufacturing methods while maintaining appropriate regulation. It launched the CGMP initiative to encourage mechanisms to help pharmaceutical manufacturers gain flexibility while retaining regulatory control. One aspect of this initiative has been the use of process analytical technologies to apply multivariate modeling and real-time analytical methods to predict and control product quality during the manufacturing process (see Guidance for Industry: PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance).
This approach acknowledges a critical reality: life-sciences manufacturers cannot rely on fixed process control schemes to create repeatable results. Variability in raw materials, equipment, and processing conditions are unavoidable and will cause variability in product quality. A more robust strategy is to develop processes with measurement and control capabilities that compensate for process variability and to foster a culture of continuous process improvements.
Process understanding — the modern basis for drug process development
Pharmaceutical products are designed to provide specific therapeutic benefits for patients. As a common example, ibuprofen reduces inflammation and pain. During the drug development process, scientists seek to understand the critical quality attributes (CQAs) needed to achieve the drug’s desired health benefits. For example, a drug’s dissolution rate affects how fast the drug is taken up into the bloodstream. Ultimately, a drug’s critical quality specifications are developed within allowed tolerances.
Once the CQA specifications are determined, scientists follow a process that uses quality-by-design principles to develop an understanding of how the critical process parameters (CPPs) affect the CQAs. They gain a science-based understanding of the relationship between the CQAs and CPPs. This is normally done through a design of experiments, ultimately developing a multivariate model that defines how variability of the CPPs affects the CQAs and the boundaries where the CPPs can operate and produce quality product. The multivariate model that defines the boundaries where the process can operate is known as the design space. It represents a significant component of process understanding. See figure 1 for an illustration of a design space.
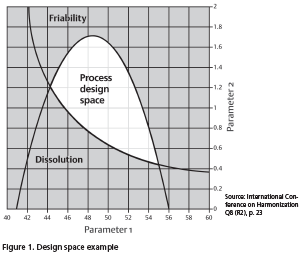 Design space example
Design space example
Once companies understand the relationships between CPPs and CQAs and define the design space, PAT-based measurement and control can be used to ensure quality and increase the overall manufacturing performance.
Using specialized tools
Implementing PAT-based measurement and control requires specialized tools. The following represent the primary tool kit for implementing PAT-based control:
- analytical instruments
- chemometric modeling tools
- software for PAT method development, data management, and systems integration
Analytical instruments used for PAT measurements are typically advanced analytical instruments using the light spectrum to characterize a quality measurement. Examples of these include:
- near-infrared spectroscopy
- Raman spectroscopy
- mass spectroscopy
- Fourier-transform infrared spectroscopy
- focused-beam reflectance measurement
The output from these types of devices is typically spectral data, which needs to be processed through a chemometric model to predict the critical quality attribute of interest. See figure 2 for an example of the spectral data output. The final piece of the puzzle is a PAT management software platform that is needed to manage data and integrate the PAT instrument with other data and control solutions.
Implementation challenges
Successfully implementing PAT is not a simple proposition. There are a variety of challenges that must be addressed. These are listed in table 1.
Specialized instruments: PAT instruments are very different than traditional temperature, pressure, and flow devices. These instruments may require specialized training to care for and maintain them in good working order. For example, some instruments require a daily performance qualification check.
Chemometric models: The development of models for calibrating instruments with spectral data output requires specialized skill sets and experience. Knowledge about the range of conditions to collect data is needed, as well as an understanding of statistics to evaluate the quality of the model. The selection of a standard chemometric modeling software package may be required. Many instrument suppliers provide software tools for this, but then different software would be used for each instrument purchased from a different supplier. In addition to the chemometric models used to predict the CQA values, process models may be developed to predict the overall behavior of the process.
PAT method development: A PAT method is the procedure required to use the PAT instrument to take the CQA measurement. This could include how many spectral readings to take, which wavelengths to read, and whether to average spectral readings over several readings. It could also be necessary to coordinate when the reading should be taken with the process. For example, perhaps CQA measurements are not taken in a bioreactor until after inoculation is completed. It is also necessary to understand the frequency with which the CQA should be measured, which is a function of the process dynamics. Specialized software for PAT deployment and data management may be used to develop and execute the PAT method (PAT management software).

Systems integration: Organizations may need to deploy PAT in a way that enables control of the process integration to a variety of software and hardware systems. Examples include:
- PAT instrument interface: The instrument itself needs to be controlled in order to execute the PAT method and to retrieve the spectral data for processing against the chemometric model.
- Process control system interface: The predicted CQA generated from the chemometric model needs to be uploaded to the process control system in real time to execute control actions based on the CQA measurement.
- Modeling environment interface: The software that processes the spectral data with the model must be initiated and triggered as part of executing the PAT method.
- Laboratory information management system (LIMS) and process historian interface: If a process model is included within the control strategy, an interface to these systems may be needed to obtain inputs to the model.
PAT data management: PAT is a data-intensive activity. The following data management challenges may be expected:
- Spectra data storage: In the pharmaceutical industry, data is never discarded. It is a standard industry practice to historicize measurement data. Spectral data presents special challenges, because it is multivariate in nature. Standard process historians are not designed for storage of spectral data.
- Model management: Because the models affect GMP, it is necessary to validate the models and keep them under revision control. A system for managing these models is required. Also, if the same models are being deployed across more than one site, an enterprise strategy to manage these models may be needed.
- PAT method management: Similarly to the models, the PAT methods must be maintained under revision control.
Process control strategy development: Measuring CQAs with PAT provides little value if it is not accompanied by a control strategy that uses it to control the process within its design space. Developing these strategies requires knowledge of process control methods as well as understanding the process dynamics.
Regulatory approval: As if overcoming the technical challenges were not enough, it may be necessary to get regulatory approval to deploy PAT as a basis for quality measurement and control. This will depend on the contents of the initial chemistry, manufacturing, and controls filing made to regulatory authorities.
Lessons learned and best practices
PAT has been implemented in many facilities. Like most innovations, “big pharma” has led the way, because it has significant resources. Now smaller producers, including contract and generic manufacturers, are following. Those now embarking on implementing PAT can take advantage of the lessons learned and best practices that have been developed to date.
This type of program has been implemented at many pharmaceutical manufacturing facilities, but there are countless ways PAT can go off the rails if not handled correctly. PAT is a complex mix of elements—technical and human—and incorrect implementation of any of these elements can cause an effort to fail. There are many best practices to ensure success, and having a qualified partner can make the process much easier. Successful PAT implementations generally follow a similar set of strategies, adjusted to a specific situation as needed (table 2):
Strategic initiative: If PAT is being implemented as a pet project at the manager or director level within process development or manufacturing, there is a significant risk of failure. A review of the challenges described above illustrates that implementing PAT and receiving its benefits will require a significant investment of time, resources, and expertise. It should only be undertaken with the sponsorship of senior management, preferably at the executive level. PAT should be managed as a program with expertise and resources leveraged across sites. Because there will be challenges, the sponsoring executive must be convinced of the benefits.
Quantified justification: As with any significant investment, it is good practice to develop a strong business case. The business case should be developed for an entire program over five years. Care must be taken to adequately cover the cost side of the analysis. Costs must include additional personnel, training, instrumentation, software, support agreements, and computing resources. The benefits side of the ledger should include:
- Increased quality: This must be quantified to nail down its financial benefits. It could result in reduction of off-spec material. Credit for improved quality may be taken as reduced cost or increased revenues, depending on whether or not the facility is limited in capacity. Improved quality could also result in better yield. Significant analysis may be required to determine how increased quality yields specific financial benefit.
- Faster product release: It is possible that PAT measurements may eliminate some post production testing and allow material to flow through the process faster.
- Reduced cycle time: In many cases, materials are over processed. Using PAT to detect the processing end point can reduce the cycle time.
- Reduced labor costs: PAT can reduce the number of samples submitted to the QC lab and reduce the lab costs.
- Energy savings: If PAT measurements enable reduction of cycle time, the cost of energy to run equipment will also be reduced.
Generating a clear business case based on tangible information and realistic assumptions is a key part of getting the organizational commitment for a successful program. Process development (PD), quality, manufacturing, and regulatory areas must ultimately be convinced of the benefits.
System architecture planning: Implementing PAT is more than purchasing analytical instruments. It should be viewed as an overall system, including instruments, modeling software, data management, process control, and integration of the components. The overall architecture should be developed from instrument to control and all the other tasks and systems needed in between. A technical strategy should be developed for accomplishing the systems integration that will be required. This must be done before putting together the business case, because these details will affect the required investment.
Cross-organizational involvement: Successful implementation of PAT is a team sport requiring the willing participation of the PD, manufacturing, quality, and regulatory departments. The following items describe the role of each department:
- Process development: Ideally, PAT should be part of the quality-by-design strategy, and all new processes should be developed to leverage available PAT technologies. When this is baked into the process development process, the effort for regulatory approval is simply part of the standard regulatory approval process for a new drug. PD is also the right department for chemometric model development, because it is where this type of resource is usually located.
- Manufacturing: Manufacturing’s role is to deploy and support the PAT systems within production. The department must have the expertise to maintain the equipment and systems, and it is responsible for ensuring that good CQA measurements are obtained and process control strategies are implemented based on the CQA measurements.
- Quality: The quality department is ultimately responsible for the release of the product, therefore it is necessary for quality to be aligned with the methodology for establishing the CQA values and the PAT methods used.
- Regulatory: The regulatory department submits documentation to regulatory authorities to get the process approved. If the PAT method is being introduced to a process that is currently producing an approved product, the regulatory department will need to make submissions to have the change in the process approved. This could include providing evidence that the new PAT method is equivalent or better than the method it is replacing.
Deploying the right skill sets: The financial analysis may show an overall reduction of labor. However, it should be understood that this is a net reduction of labor. Some additional resources may be needed to handle some of the specialized tasks PAT involves. Supporting and maintaining PAT instruments will require additional workload and skills from the instrument technicians. Additional skills and effort may be required for model development, process control strategy development, software systems support, and data management. While none of these areas need to require a dedicated head count, the additional workload and skill sets cannot be viewed as just another part-time job heaped upon already overloaded people. This will cause failure of the program. It does make sense to rely on contractors with expertise to start the program. However, it is not possible to outsource the entire program. An appropriate level of expertise is needed in house even to provide oversight of contractors.
Program plan: Before developing the business case, develop a program plan. This plan should include a phased approach toward implementing the architecture, a human resource plan to ensure the right skills are in place, and a roll-out plan of how and when PAT will be deployed in production. It is possible the program plan could establish that PAT will only be included for new products to reduce the regulatory burden of approving changes to processes in production. Or, it may be decided the best place to start is a high-volume product in production that will deliver lots of benefit.
Deploying PAT as a strategy to increase quality and improve financial performance of drug product and drug substance manufacturing is proven. Many papers are available highlighting successful implementations and the benefits realized. The FDA and other regulatory agencies promote the adoption of new technologies, and the industry is being encouraged to move forward with PAT and other advanced methods for process monitoring and control. As companies decide to adopt these technologies, they should take care to execute investments as part of a strategic initiative and have a realistic understanding of the challenges. Although this is not rocket science, it is chemical science. Obtaining overall organizational commitment and applying good planning and resources are the keys to success.
Note: Chemometrics is the chemical discipline that uses mathematical and statistical methods to design or select optimal procedures and experiments, and to provide maximum chemical information by analyzing chemical data.
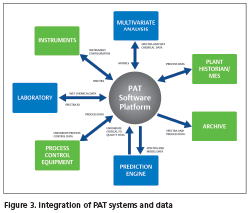 Integration of PAT systems and data
Integration of PAT systems and data
About the Author Jonathan Lustri is a life sciences industry consultant for Emerson Automation Solutions. He has a BS in chemical engineering from the University of Texas at Austin and 35 years of experience in process engineering, development, and automation. Lustri has worked for Emerson’s life sciences industry group for 15 years in a variety of capacities. He currently specializes in helping Emerson’s life sciences industry customers identify methods for using PAT and other process controls to improve quality and throughput. Lustri also has a Lean Six Sigma Black Belt certification from Villanova University.
Jonathan Lustri is a life sciences industry consultant for Emerson Automation Solutions. He has a BS in chemical engineering from the University of Texas at Austin and 35 years of experience in process engineering, development, and automation. Lustri has worked for Emerson’s life sciences industry group for 15 years in a variety of capacities. He currently specializes in helping Emerson’s life sciences industry customers identify methods for using PAT and other process controls to improve quality and throughput. Lustri also has a Lean Six Sigma Black Belt certification from Villanova University.
A version of this article also was published at InTech magazine.




